Enzyme Regulators And Inhibitors
Enzyme Regulators And Inhibitors Assignment Help | Enzyme Regulators And Inhibitors Homework Help
Enzyme Regulators and Inhibitors
Some kind of regulatory mechanisms exist within the cell to control enzyme catalyzed reactions. These regulatory mechanisms operating at several levels not only modulate the catalytic activity, but also inhibit the enzyme production if it is in the interest of cell. Generally the enzymes are modulated in the cell by low molecular weight inhibitors which may or may not be present at all times. Inhibitors also provide a means to study the functional groups at the active site.Certain compounds, called antimetabolites, when added to the medium combine with the enzyme reversibly or irreversibly to block the production of end products. Such substances are called inhibitors and include drugs, antibiotics, poisons, and certain metabolites. Inhibition occurs in a variety of ways, out can be broadly divided into two types, reversible and irreversible.
Reversible Inhibition
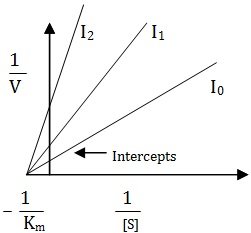
Many inhibitors reversibly bind with the enzyme molecule affecting the equilibrium constant of the reaction. Several types of such inhibitions are known and can be distinguished with the help of Lineweaver-Burk plots of enzyme kinetics.Competitive Inhibition
When the inhibitors compete with the substrate of an enzyme, either by occupying the catalytic site or by altering the enzyme conformation that is unfavorable for substrate binding, inhibition is called competitive. Inhibition of this nature can be reversed by increasing the substrate concentration. Such inhibitors show structural resemblance with the substrate, and thus combine with the active site of the enzyme, normally occupied by the substrate.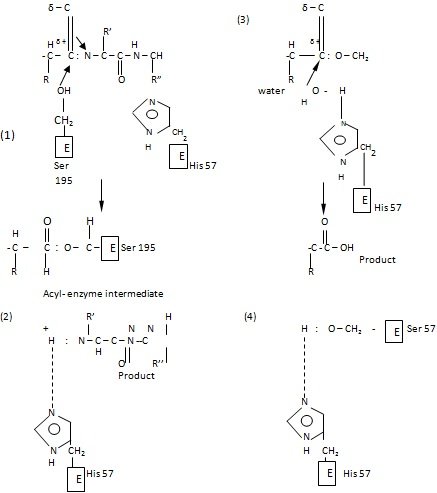
Non –Competitive Inhibiton
In this case the inhibitor binds the active site in such a way that the substrate is still bound to the enzyme, but reaction is prevented from occurring. However, the level of inhibition is governed by concentration of inhibitor.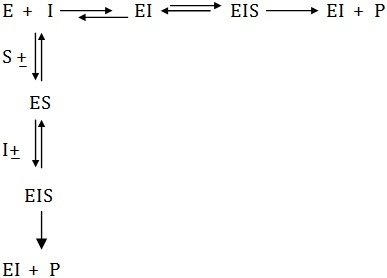
The equation for non-competitive inhibition
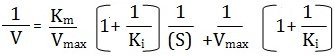
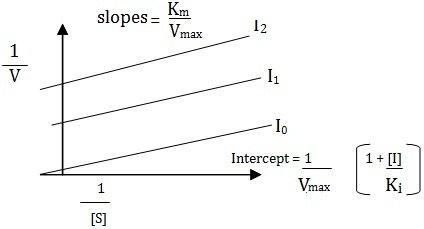
Uncompetitive Inhibition
Some inhibitors can combine reversibly with ES-complex only, hence are called uncompetitive inhibitors have no affinity with the (S) and bear no resemblance to it and therefore yield no products. This typeof inhibition is found in multisubstrate reactions. The slope of the plot is given by Km/Vmax and the equation is
Irreversible Inhibition
Irreversible inhibition is brought about when an inhibitor covalently binds with the functional group at the active site and physically blocks it. Such inhibitions do not follow Michaelis-Menten kinetics.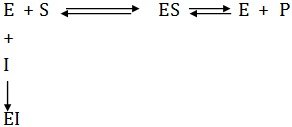
Compounds such as iodoacetate (CH2I.COOH), mercurials, or organophosphorus compounds (diisopropyl flurophosphate) act as irreversible inhibitors and block the functional-SH group of the enzyme to form covalent derivatives.

The enzyme is totally inactivated and the inhibitor cannot be released by any means. The inhibitor diisopropyl fluorophosphate is nerve poison, and a specific inhibitor of esterases hydrolyzing esters.
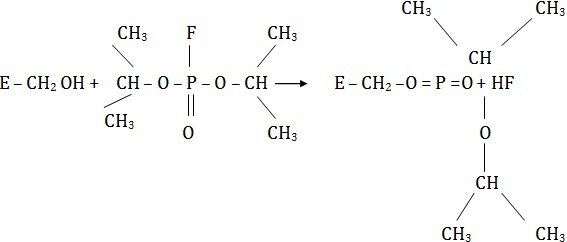
The enzyme is totally inactivated and the inhibitor cannot be released by any means. The inhibitor diisopropyl fluorophosphate is nerve poison, and a specific inhibitor of esterases hydrolyzing esters.

For more help in Enzyme Regulators And Inhibitors please click the button below to submit your homework assignment