Angular Wave Function
Angular Wave Function Assignment Help | Angular Wave Function Homework Help
Angular Wave Function
The angular part of the wave function depends on 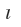 and m values and is often referred as atomic orbital. The shapes of these atomic orbital’s depend upon angles
and m values and is often referred as atomic orbital. The shapes of these atomic orbital’s depend upon angles 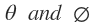 which determine geometry of the atom. Mathematical expressions for angular wave functions of s and p orbital’s are given in.
which determine geometry of the atom. Mathematical expressions for angular wave functions of s and p orbital’s are given in.
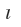 and m values and is often referred as atomic orbital. The shapes of these atomic orbital’s depend upon angles
and m values and is often referred as atomic orbital. The shapes of these atomic orbital’s depend upon angles | Quantum Nos. |
Quantum Nos m |
Orbitals |
Angular Wave Function |
| 0 1 1 1 |
0 0 1 -1 |
S pz px py |
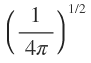 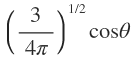 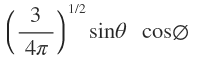 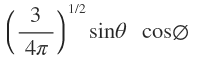 |
The expression for s-orbital has no angular
It may be summarized that
(i) radial part of wave function R ( r) depends upon quantum number n, 1 and gives the distribution of electron w.r.t its distance form the nucleus.
(ii) the point at which R (r) changes sign or R (r) = 0 is called a nodal point.
(iii) s-orbital’s have (n-1) nodes; p-orbital’s (n-2) nodes , d-orbital’s (n-3) nodes, and f-orbital’s (n-4) nodes.
(iv) the angular wave function depends on
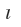 and m values and is often referred as atomic orbital’s.
and m values and is often referred as atomic orbital’s.(v) Shapes of atomic orbital’s depends on angles
(vi) s-orbital shave no angular dependence and hence spherically symmetrical.
For more help in Angular Wave Function click the button below to submit your homework assignment