Energy Level Of Hydrogen
Energy Level Of Hydrogen Assignment Help | Energy Level Of Hydrogen Homework Help
Energy Level of Hydrogen
Hydrogen atom is the simplest atom having only one electron which normally occupies the s-orbital of principal quantum number 1. In the excited state, however, the electron can jump to orbital’s corresponding to principal quantum number 2,3,4 etc. It has been calculated form Schrodinger wave equation that eh energy of the electronic a hydrogen atom ( Z =1) is given by the following expression :
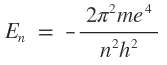
Where m is the mass of electron, e is the change on the electron, h is the Planck’s constant and n is the principal quantum number.
In this expression all the faction on the right hang side except n are constant. Substituting the values f these faction the expression gets reduced to :
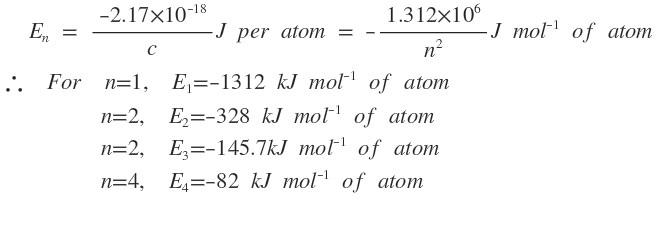
It may be noted that the electronic energy levels in a hydrogen atom depend only upon the value of principal quantum number. Thus 2s and 2p have the same energy; 2s, 3p and 3d have equal energies; 4s , 4p 4d and 4f have equal energies and so on .
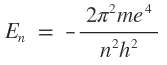
Where m is the mass of electron, e is the change on the electron, h is the Planck’s constant and n is the principal quantum number.
In this expression all the faction on the right hang side except n are constant. Substituting the values f these faction the expression gets reduced to :
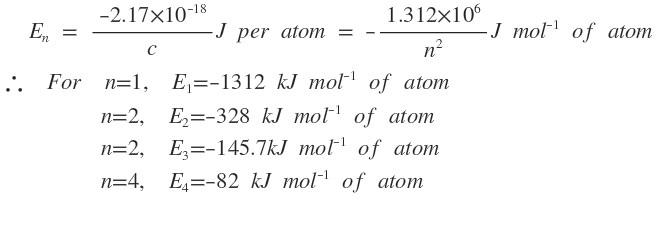
It may be noted that the electronic energy levels in a hydrogen atom depend only upon the value of principal quantum number. Thus 2s and 2p have the same energy; 2s, 3p and 3d have equal energies; 4s , 4p 4d and 4f have equal energies and so on .
Energy level diagram for hydrogen atom
In case of other ions such as He+ and Li2+ which are like hydrogen i.e. have only one electron around the nucleus , in their sub shells have equal energies, However, their energies do depend on nuclear change and are calculated using the equation.
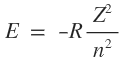
where R = Rydberg constant
n = Principal quantum number
Z = atomic number = nuclear charge
For more help in Energy Level of Hydrogen click the button below to submit your homework assignment