Multi Electron System
Multi Electron System Assignment Help | Multi Electron System Homework Help
Multi-Electron System
The Schrödinger wave equation has been solved exactly for the hydrogen atom only, that is, an atom having one proton and one electron. This treatment can also be applied to one-electron systems such as He+,Li2+ and Be3+ after taking into account the appropriate value of the nuclear change Z = 2,3 and 4, respectively. However, difficulties aeries when we attempt to apply it to atoms containing more than one electron. In such atoms, besides attraction of each electron for the nucleus, there are inter-electronic repulsions which make the exact solution of a wave equation imposable and approximation method developed by Harte and Flock is called the self-consistent treatment.
This treatment simplifies the problem to that of a single electron moving in a spherically symmetrical field of a force provided by the uncles and the averaged effect of all other electrons. The final atomic orbital’s are given as numerical expressions which are difficult to convert to a physical mode, However, these orbital’s are not medially different form those of hydrogen atom but all the orbital’s are same what contracted due t the increased nuclear change.
This treatment simplifies the problem to that of a single electron moving in a spherically symmetrical field of a force provided by the uncles and the averaged effect of all other electrons. The final atomic orbital’s are given as numerical expressions which are difficult to convert to a physical mode, However, these orbital’s are not medially different form those of hydrogen atom but all the orbital’s are same what contracted due t the increased nuclear change.
A typical result of these lengthy calculations is shown in where radial distribution function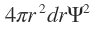 is plotted against r for the sodium atom
is plotted against r for the sodium atom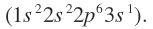
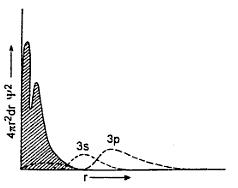
Radial Probability distribution of curve for Na.
The shaded portion shows the electron distribution in Na+ and the dotted line marked as 3s shows that distribution curve for the outermost electron in the neutral Na atom. The diagram show that in Na+ ion the electron change distribution effectively forms two concentric shells quire close to the uncles, indicating somewhat contraction of inner shells and the effectiveness with which the inner electrons careen the uncles form the outer electrons. The 3s electron venue has a significant probability of being close to the uncles underneath the inner electron s(of ) as shown . That is, inner electron do not shield the nucleus completely form the 3s-electrons. The 3p-electron curve (bolder dots) is shielded a little more, effectively (than the 3s curve) by the Na+ core and so on. It may be inferred form the radial distribution plot that the degree of penetration deceases in the order 3s > 3p > 3d. This reveals that in multlectron atoms, the inner electrons is general are closer to the uncles and the outer enlacing penetrate the inner core of electrons to some extent ant eh degree of penetration (for a given n) decreases in the order s > p >d >f.
For more help in Multi-Electron System click the button below to submit your homework assignment
The shaded portion shows the electron distribution in Na+ and the dotted line marked as 3s shows that distribution curve for the outermost electron in the neutral Na atom. The diagram show that in Na+ ion the electron change distribution effectively forms two concentric shells quire close to the uncles, indicating somewhat contraction of inner shells and the effectiveness with which the inner electrons careen the uncles form the outer electrons. The 3s electron venue has a significant probability of being close to the uncles underneath the inner electron s(of ) as shown . That is, inner electron do not shield the nucleus completely form the 3s-electrons. The 3p-electron curve (bolder dots) is shielded a little more, effectively (than the 3s curve) by the Na+ core and so on. It may be inferred form the radial distribution plot that the degree of penetration deceases in the order 3s > 3p > 3d. This reveals that in multlectron atoms, the inner electrons is general are closer to the uncles and the outer enlacing penetrate the inner core of electrons to some extent ant eh degree of penetration (for a given n) decreases in the order s > p >d >f.
For more help in Multi-Electron System click the button below to submit your homework assignment