Chemical Bondings
Chemical Bondings Assignment Help | Chemical Bondings Homework Help
Chemical Bondings
Several types of chemical bonds are found in biological molecules that are responsible for stability and the required conformation that determine the behavior of these molecule. A bond is any force which holds two atoms together. Carbon is linked to H,O,N,S and p by covalent bonds which are formed when two atoms share a pair of electrons. We describe the bonds involved in biological molecules.
Covalent Bonds
Covalent bond is formed by electron pair distributed over two or more atoms ,i.e, it is a shared electron pair. These are stable bonds which hold the adjacent atoms together. The energy needed to break such bonds ranges from 50 to 100 kcal mole-1. In the following example each pair shared electrons is covalent bond:
Covalent Bonds
Covalent bond is formed by electron pair distributed over two or more atoms ,i.e, it is a shared electron pair. These are stable bonds which hold the adjacent atoms together. The energy needed to break such bonds ranges from 50 to 100 kcal mole-1. In the following example each pair shared electrons is covalent bond:
H
. .
H: N :H
. .
H
. .
H: N :H
. .
H
Dipoles
Any molecule with positive and negative ends is a dipole. The forces of attraction between two dipoles are responsible for the orientation of molecules with respect to each other, which helps in arrangement of the molecules. A dipole may also induce a dipole in a nonpolar molecule. Two nonpolar molecules are also able to have mutual attraction because of mutually induced dipoles.
lonic Bonding
This is due to the attraction between atoms or groups of opposite charge (+ and -). The bonding occurs in crystals and salts that are ionized into ions when dissolved in water. For example, NaCl is salt composed of Na+ (cation and Cl¯ (anion)

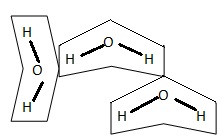
Hydrogen Bond
Hydrogen bonds are weak as compared to covalent bonds the energy required to break them ranges between 0.5 to 12 kcal mole-1 .The attractions of a hydrogen in dipole for a negative atom in another dipole is called a hydrogen bond.
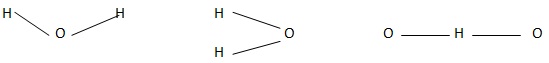
The hydrogen bonds in biological molecules have assumed greater significance since they are primarily required for the specificity of interaction and conformations of macromolecules.
Dipole –Dipole Bond
This refers to the attraction between the positive end of one dipole and the negative end of another dipole .one of the example of dipole-dipole bonds is the hydrogen bond. Ordinary hydrogen has a valence of one and it can share an electron pair in forming a bonafide covalent bond or it can gain an electron to form the hydride ion H+ . Hydrogen atom carries a relatively high partial charge when covalently bonded to an electronegative element, such as oxygen and nitrogen. Because it has an extremely small proton it can approach a second atom with partial negative charge. This closeness allows the formation of a dipole-dipole bond, sufficiently strong but weaker than a normal covalent bond.
Non-polar Groups (Hydrogen Bonds)
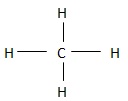
Non-polar groups are also called hydrophobic groups and all hydrocarbons have them. The hydrophobic groups are clustered together through hydrophobic bonding that takes place in water. In the molecule shown below, the hydrogen covalently bonded to carbon in non- polar and does not bear any charge due to equal electron sharing. It is therefore a non- polar group and does not form hydrogen bonds.
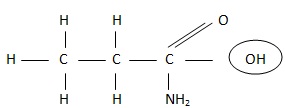
Polar Groups
The polar groups have a high attraction for water and form hydrogen bonds with it. These polar groups increase the solubility of a compound in water.

The polar groups is shown in a circle.
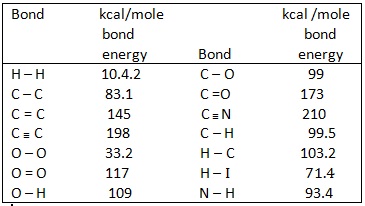
Bond Energies
atoms contain more energy compared to molecules composed of atoms.The atoms are held together by forces which can be estimated . Bond energies or the heat of formation of bonds represent the energy lost by the molecular system when bonds are formed between atoms. In order to break these bonds the same amount of energy is required. Thus the total energy of the molecule is the sum of bond energies and dissociation energy and is measured in terms of kilocalories needed to form a bond.
For more help in chemical bondings click the button below to submit your homework assignment