Classification Of Amino
Classification Of Amino Assignment Help | Classification Of Amino Homework Help
Classification Of Amino Acids
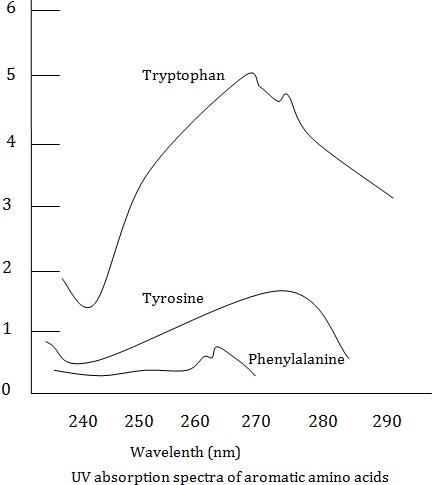
All the naturally occurring amino acids are classified on the basis of their R groups or side chains : (a) amino acids with non –polar R groups, (b) amino acids with polar but unchanged R groups (C)
Amino acids with positively charged R groups and (d) amino acids with negatively charged R groups. The classification of amino acids along with the trivial names as used in protein chemistry.
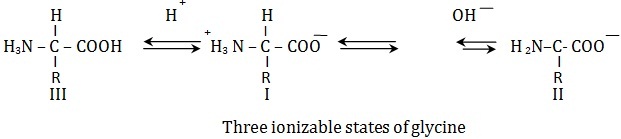
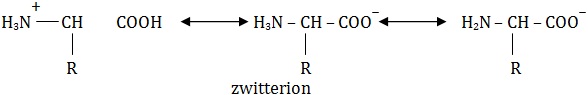
Zwitterionic Nature of Amino Acids
Amino acids contain acidic and basic groups and can both donate and accept protons, hence they are said to be amphoteric. The carboxyl group is acidic and can donate a proton by dissociation. The amino group, being basic, can accept a proton. When the groups are ionized, the amino acid is said to be zwitterion. Amino acid in aqueous solution are in zwitterionic form.
Amino acids an Electrolytes
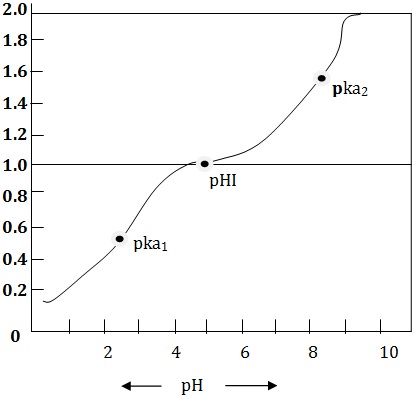
Amino acids are ionized to various degrees depending upon the ionizable groups they possess. The dissociable constant, pK values, are determined for the ionizable groups. If a solution of glycine hydrochloride (0.2 M glycine solution with 0.2 m HCT is titrated with 0.1 M NaOH solution and the pH be determined. The first pK1 value is obtained when the COOH group is ionized and the second pK2 value is observed when ammonium ion is dissociated. The result can be graphically plotted in the form of a biphasic curve.