Enzyme Kinetics
Enzyme Kinetics Assignment Help | Enzyme Kinetics Homework Help
Enzyme Kinetics
The Michaelis-Menten equation can be derived by making certain assumptions employing Briggs and Haldane derivation for steady state kinetics. In an enzyme catalyzed reaction, the enzyme E must combine with its substrate S to form an ES-complex which then breaks down to yield the product and the enzyme is set free to be reused.Where k1,k2,k3 and k4 are rate constants for each reaction.
Before deriving the equation, let us make few generalizations
2. The effect of substrate concentration on the velocity of enzyme reaction can be visualized by having three points A, B and C on the hyperbola.
3. A represents the stage when the velocity(v) of reaction is dependent on the substrate concentration.
4. At B, substrate concentration is equal to km, hence the velocity is half of Vmax.
5. At point C, substrate concentration is much greater than the Km value hence the velocity is maximum (Vmax). The enzyme at this stage is fully saturated with the substrate and any increase in its concentration will have no effect.
Application of Michaelis-Menten Equation
The Michaelis-Menten equations is sufficient to describe most of the enzyme catalyzed reactions. It can be utilized to determine Km at various substrate concentrations and the values can be used in predicting rate –limiting steps. Therefore, Vmax and Km should be carefully determined .However, an important method in determining these values precisely is to take reciprocal of substrate concentration and velocity of reaction to draw Lineweaver-Burk polts. One of the major application of these plots is to study enzyme inhibition.
Line-Burk Plot
The Michaelis –Menten equation can be transformed by taking the reciprocal of both sides of the equation: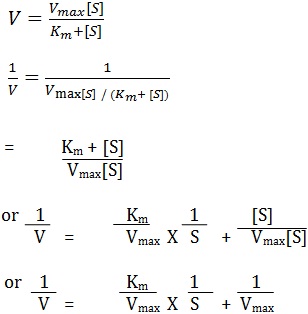
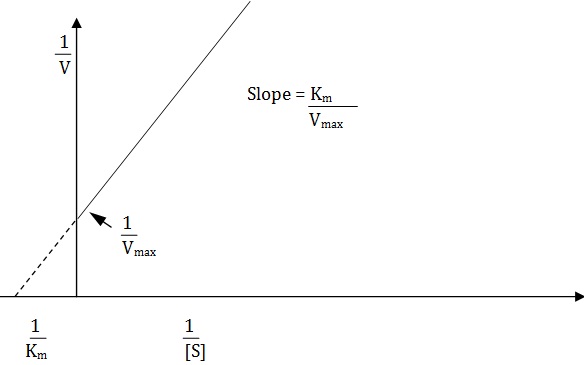
If a double reciprocal plot is drawn by taking 1/v values on the ordinate and 1/[s] values on the abcissa, a straight line is obtained from which Km values can be calculated the slope of the line is represented by Km/Vmax and the 1/ Vmax.
Such plots are handy in distinguishing the nature of inhibitors.
For more help in Enzyme Kinetics please click the button below to submit your homework assignment