Enzyme Specificity
Enzyme Specificity Assignment Help | Enzyme Specificity Homework Help
Enzyme Specificity
The effectiveness of enzyme is due to specific binding and location of specific groups at the active site which are also responsible for its specificity. Till date about 1200 different enzyme have been isolated and studied and each one of them has been found to possess a range of specificities. This means that enzyme-substrate interactions are selective in nature. Enzymes can therefore be divided into a number of types based on the degree of specificity they exhibit.
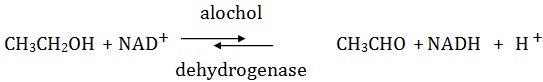
Although this enzymes show prefers ethyl alcohol, it can also act on long chain alcohols, but with less efficiency.
Absolute specificity
It is exhibited by some enzymes where each can act only on one substrate. Urease shows absolute specificity towards its substrate, urea, and any modification in the substrate molecule will render the enzyme ineffective.Broad Specificity
Some enzymes act on a range of substrates related to each other, showing broad specificity. Enzymes of this type are economical for the cell because this behavior minimizes the number of enzyme.Trypsin and carboxypeptidase are included in this category. Carboxypeptidase acts on from the C-terminal, irrespective of the nature of amino acids.Group Specificity
Certain enzymes have a preference for a specific organic group present on the substrate molecules, e.g., alcoholic dehydrogenase acts on alcohols.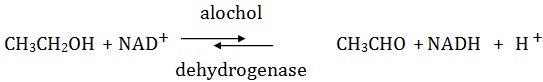
Stereochemical specificity
Certain enzymes show optical specificity i.e., they are able to discriminate between their optical isomers which are related substrates. For instance, an L-amino acid oxidase, a flavin enzyme, will act on an L-isomer only and not on a D-isomer.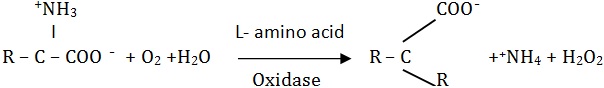
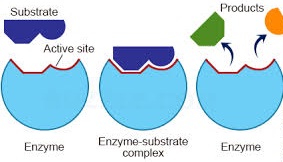
Enzyme-Substrate Interaction
It is generally believed that the functional groups at the active site are fixed points of attachment with the substrate. In other words, the active site is a rigid structure in space, in which the substrate fits itself. Emil Fischer postulated a lock and key hypothesis to explain this interaction between the enzyme molecule and substrate. The model visualizes the enzyme molecule as a rigid structure having a fixed substrate binding site.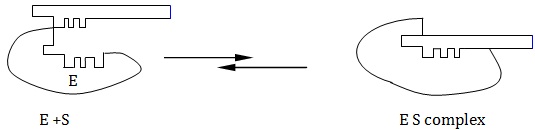
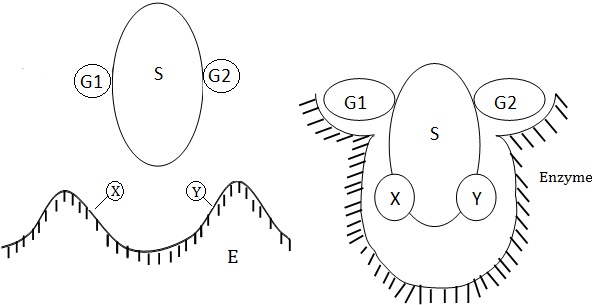
The concept of lock key was later modified by Daniel Koshland in 1968, who proposed an induced fit model according to which an enzyme molecule is a flexible structure which undergoes conformation change induced by the substrate molecule, so that a proper alignment between the enzyme molecule and substrate is established conformational changes in the enzyme molecule have been observed by X-ray diffraction studies.
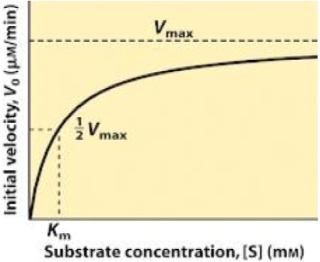
Enzyme-substrate interaction could be studied with the help of where substrate concentration has been shown on the X-axis and velocity of reaction on Y-axis. When the enzyme catalyzed reaction is studied at various substrate concentrations, a hyperbolic curve is obtained. Three points, namely A, B and C can be examined on the curve, which represent three situations. At point A, substrate concentration (S) is very low and the rate of reaction is directly proportional to substrate concentration. As the increases reaching a maximum (Vmax) as shown at point C in half of maximal velocity. At high substrate concentration the rate of reaction becomes independent of its concentration, and the enzyme appears to be fully saturated with the substrate.
The enzyme catalyzed reaction proceeds in two steps:

In the process of enzyme first combines with the substrate forming an ES-complex, which subsequently breaks down into product (P) with the recovery of enzyme(E).
The enzyme catalyzed mechanism was quantified by Michaelis and Menten in 1913, who applied the law of mass action to the formation of ES-complex and developed on equation commonly called Michaelis-Menten equation.
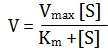
Where V is velocity of the reaction Vmax the maximal velocity, S the substrate concentration , and Km is the Michaelis-Menten,s constant in moles per liter solution. Km represents the S concentration at which the velocity is half of Vmax.
For more help in Enzyme specificity please click the button below to submit your homework assignment