Flavin Nucleotides
Flavin Nucleotides Assignment Help | Flavin Nucleotides Homework Help
Flavin Nucleotides
Falvin nucleotides or flavoproteins are coenzymes which consist of riboflavin (vitamins B2) as an active component involved in hydrogen transfer reactions. Riboflavin occurs in many plants and bacteria which was isolated successfully by G . R . Kuhn and T. Wagner- jauregg in 1933. Riboflavin is derivative of isoalloxazine, having a pteridine ring fused with a benzene ring with a side chain of C5- polyhydroxy group, a derivative of sugar ribitol. Riboflavin is constituent of coenzymes which catalyze oxidation reduction reactions. The oxidized form has an intense yellow color, and hence is also known as the “yellow enzyme”. The reduced form is colorless. The two coenzymes in which riboflavin is present are known as flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD+).
Biochemical function Riboflavin is a constituent of enzymes called flavoprotein dehydrogenases, which directly removes hydrogen and the associated electrons from the fuel molecules and transfers them to ubiquinine. An important example of oxidation reduction reaction is the oxidation of succinate to fumarate by FAD.
Biochemical function Riboflavin is a constituent of enzymes called flavoprotein dehydrogenases, which directly removes hydrogen and the associated electrons from the fuel molecules and transfers them to ubiquinine. An important example of oxidation reduction reaction is the oxidation of succinate to fumarate by FAD.
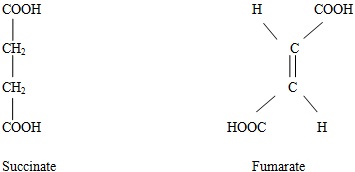
Succinate dehydrogenase is found in the inner mitochondrial membrane that contains Fad and two iron sulfide proteins. The isoallaoxazine ring functions as a reversible redox system in which hydrogen is added at N1 and N-10 positions.
For more help in Flavin Nucleotides please click the button below to submit your homework assignment