Hydrogen Ion Concentration
Hydrogen Ion Concentration Assignment Help | Hydrogen Ion Concentration Homework Help
Hydrogen Ion Concentration
Aqueous solution of acids and bases are expressed in terms of H and OH ion concentration. The term pH was introduced by Sorensen in 1909 who defined it as the negative logarithm of H+ ions, therefore
pH = - log OH¯
Similarly pOH is expressed as –log OH¯.
Ion product of water at room temperature is 1 x 10-14,the pH of pure water is 7 because H+ = 10 x 10-7 and –log(1x10-7)=7. It follows that pH = pOH=14, that is why when pH is less than 7, solution is acidic and when pH is more than 7, the solution is basic.
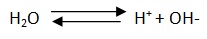
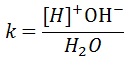
As the concentration of water is very large compared to H+ , it may be taken constant. The ionic product of water Kw is defined as Kw = [H+][H¯].
For more help in Hydrogen Ion Concentration click the button below to submit your homework assignment
For more help in Hydrogen Ion Concentration click the button below to submit your homework assignment