Mechanism Of Catalysis
Mechanism Of Catalysis Assignment Help | Mechanism Of Catalysis Homework Help
Mechanism of Catalysis
Many enzymatic reactions are reversible, hence they are capable of catalyzing forward as well as backward reactions. The interaction between the enzyme molecule and its substrate is highly specific and involves the active site responsible for catalysis. An enzyme molecule may have one or more such regions composed of a few functional groups of side chains or amino acid residues, and also some amide groups of the polypeptide. Some enzymes possess a prosthetic group for catalysis which may also be a part of the active site. According to recent findings the functional groups at the active site have a fixed position, and as the substrate and when this happens a part of the polypeptide chain shift to form a bond with it. This corresponds to the induced-fit hypothesis enunciated by Koshland.
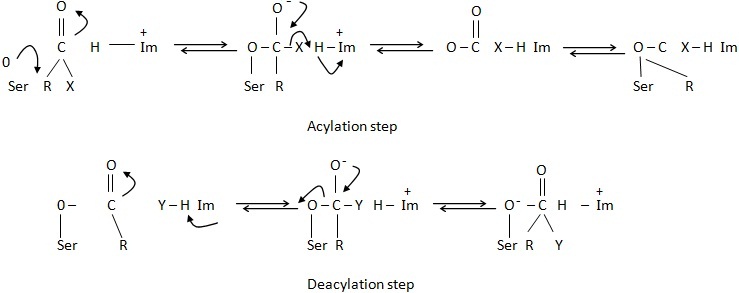
Chymotrypsin is the best studied enzyme and has been extensively used to demonstrate the mechanism of catalysis. The geometry of the active site has been probed by X-ray diffraction analysis. With the help of specific blocking agents (inhibitors) it is revealed that the active site of chymotrypsin consists of side chains residue (no. 195) and histidine residue (no .67), which are located close to each other. The enzymes participates in the transfer of acyl group from a number of donors. The mechanism of catalysis is a two step process, while the enzyme substrate interaction shows the formation of a covalent acyl-enzyme intermediate.
For more help in Mechanism of Catalysis please click the button below to submit your homework assignment
Chymotrypsin is the best studied enzyme and has been extensively used to demonstrate the mechanism of catalysis. The geometry of the active site has been probed by X-ray diffraction analysis. With the help of specific blocking agents (inhibitors) it is revealed that the active site of chymotrypsin consists of side chains residue (no. 195) and histidine residue (no .67), which are located close to each other. The enzymes participates in the transfer of acyl group from a number of donors. The mechanism of catalysis is a two step process, while the enzyme substrate interaction shows the formation of a covalent acyl-enzyme intermediate.

In the proposed mechanism of action of chymotrypsin, serine and histidine carry out a nucleophilic attack on the substrate. In the first step, the eletronegative oxygen atom of serine carries out a nucleophilic attack on the electropositive carbon of the carbonyl function (C =O) of the substrate cleaving the peptide bond so as to bring the imidazole ring of the histidine residue nearer to serine. This is accompanied by removal of a proton from serine, which is donated to the nitrogen atom of the cleaved peptide bond and the enzyme then covalently binds with the substrate. In the second step the electronegative oxygen atom of a water molecule displaces the acyl group from the enzyme, making the enzyme free. Histidine also plays a role in proton transfer and donated to the serine residue.