Atomic Structure
Atomic Structure Assignment Help | Atomic Structure Homework Help
Atomic Structure
Based on the quantum concept of Max Planck (1900) and Rutherford’s (1911) model of the nuclear atom, Niles Bohr proposed a revolutionary idea known as Bohr model of structure of atom in 1913. Bohr assumed
(i) electrons keep on revolving around the nucleus in only certain permissible orbits where it does no gain or lose energy
(ii) the angular momentum of the electron (mvr can take on only certain permitted values .
This requirement, known as the quantum condition is expressed as
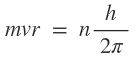
Where n= principal quantum number (1,2,3,....)
and h =plank constant.
Using those assumptions (i) and (ii)- stable orbits and quantization, respectively- Bohr model described accurately the following facts about the structure of atom:
(i) The correct radius and exact energy of electron in hydrogen atom.
(ii) The atomic spectrum of hydrogen atom.
With little modification form Somerfield, Bohr theory was satisfactorily extended to some other multi-electron atoms.
However, serious faculty in Bohr model become apparent in multi-electron atoms and some of the shortcomings in Bohr theory are listed below:
(i) It could not be applied successfully to atoms other than hydrogen without drastic and some what unsatisfactory modifications.
(ii) It is based on the laws of classical physics which cannot describe properly the motion of extremely small particles such as electrons.
(iii) It could not explain the splitting of spectral lines in a magnetic field called Zeeman effect.
(iv) It could not explain the splitting of spectral lines in an electric field known as Stark effect.
(v) It could not explain the shapes of molecules formed by the combination of atoms
(vi) It is not in accordance with eh Heisenberg’s Uncertainty principle.
In view of the above, a more sophisticated theory about the structure of atom was developed. This is known as wave mechanical model of the atom.
For more help in Atomic Structure click the button below to submit your homework assignment
(i) electrons keep on revolving around the nucleus in only certain permissible orbits where it does no gain or lose energy
(ii) the angular momentum of the electron (mvr can take on only certain permitted values .
This requirement, known as the quantum condition is expressed as
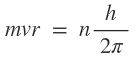
Where n= principal quantum number (1,2,3,....)
and h =plank constant.
Using those assumptions (i) and (ii)- stable orbits and quantization, respectively- Bohr model described accurately the following facts about the structure of atom:
(i) The correct radius and exact energy of electron in hydrogen atom.
(ii) The atomic spectrum of hydrogen atom.
With little modification form Somerfield, Bohr theory was satisfactorily extended to some other multi-electron atoms.
However, serious faculty in Bohr model become apparent in multi-electron atoms and some of the shortcomings in Bohr theory are listed below:
(i) It could not be applied successfully to atoms other than hydrogen without drastic and some what unsatisfactory modifications.
(ii) It is based on the laws of classical physics which cannot describe properly the motion of extremely small particles such as electrons.
(iii) It could not explain the splitting of spectral lines in a magnetic field called Zeeman effect.
(iv) It could not explain the splitting of spectral lines in an electric field known as Stark effect.
(v) It could not explain the shapes of molecules formed by the combination of atoms
(vi) It is not in accordance with eh Heisenberg’s Uncertainty principle.
In view of the above, a more sophisticated theory about the structure of atom was developed. This is known as wave mechanical model of the atom.
For more help in Atomic Structure click the button below to submit your homework assignment