Boric Acid
Boric Acid Assignment Help | Boric Acid Homework Help
Boric Acid
It is also known as orthopedic acid.
Preparation
It is obtained by adding calculated quantity of hydrochloric acid to a hot concentrated solution of borax (Na2B4O.10H2O). The resulting solution is allowed to cool when crystals of boric acid are obtained.
Na2B4O7 + 2HCI + 5H2O → 2NaCI + 4H3BO3
Properties
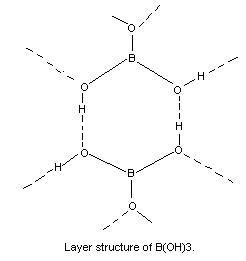
(i) It is a flaky, white crystalline substance in which BO3 units are joined by unsymmetrical H bonds as shown in. There is only weak interaction between the different layers which accounts of its low density, 1.48 g cm-3 and easy cleavage.
(ii) It is a weak monobasic acid and its acid properties are manifested in terms of OH- ion acceptance (as a Lewis acid) rather than H+ (proton) donation. i.e., it does not act as a Bronsted acid.
B(OH)3 + 2H2O <==> H3O+ + B(OH)4-; pK = 9.25
(iii) Partial dehydration of boric acid yields metaboric- acid, HBO3 which exists in different crystalline forms.
Rapid
HBO2 → B(OH)3 → HBO2 → HBO2
(cubic) 1700C 1400C (molnoclinic) (quenching) orthorhombic)
Boric acid on heatin to red heat gies B2O3
red not
2H3BO3 → B2O3 + 3H2O
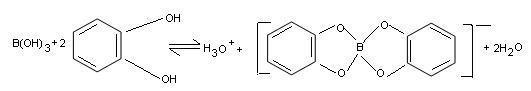
(iv) Being a weak acid, it is difficult to titrate boric acid against sodium hydroxide as the end-point cannot be located correctly. However, it can be successfully titrated in the presence of polyhydroxy alcohols (e.g. glycerol. mannitol, catechol or sugar) which greatly enhance the acidity of boric acid. Boron is complexed by these compoin. These complex ions cannot interact with H+ as boron atom has already acquired its maximum covalence of four. Consequently, boric acid in presence of polthydroxy alcohols can be titrated against strong bases like NaOH to a definite end point.
(v) When boric acid is heated with C2H5OH and conc. H2SO4, volatile triethyborate obtained. This ester burns with a green flame.
Cone H2SO2
H3BO3 + 3C2H5OH → (C2H5)3 BO3 + 3H2O
(vi) When boric acid is reacted with KHF2 (KF,HF), KBF4 salt is produced.
2KHF2 + H3BO3 → KBF4 + KOH + 2H2O
Potassium
Fluoroborate
(vii) When H3BO3 is heated with CaF2 and conc. H2SO4, boron trifluoride is formed
3CaF2 + 3H2SO4 + 2H3BO3 → 3CaSO4 → 3CaSO4 + 2BF3 +6H2O
For more help in Boric Acid click the button below to submit your homework assignment
Preparation
It is obtained by adding calculated quantity of hydrochloric acid to a hot concentrated solution of borax (Na2B4O.10H2O). The resulting solution is allowed to cool when crystals of boric acid are obtained.
Na2B4O7 + 2HCI + 5H2O → 2NaCI + 4H3BO3
Properties
(i) It is a flaky, white crystalline substance in which BO3 units are joined by unsymmetrical H bonds as shown in. There is only weak interaction between the different layers which accounts of its low density, 1.48 g cm-3 and easy cleavage.
(ii) It is a weak monobasic acid and its acid properties are manifested in terms of OH- ion acceptance (as a Lewis acid) rather than H+ (proton) donation. i.e., it does not act as a Bronsted acid.
B(OH)3 + 2H2O <==> H3O+ + B(OH)4-; pK = 9.25
(iii) Partial dehydration of boric acid yields metaboric- acid, HBO3 which exists in different crystalline forms.
Rapid
HBO2 → B(OH)3 → HBO2 → HBO2
(cubic) 1700C 1400C (molnoclinic) (quenching) orthorhombic)
Boric acid on heatin to red heat gies B2O3
red not
2H3BO3 → B2O3 + 3H2O
(v) When boric acid is heated with C2H5OH and conc. H2SO4, volatile triethyborate obtained. This ester burns with a green flame.
Cone H2SO2
H3BO3 + 3C2H5OH → (C2H5)3 BO3 + 3H2O
(vi) When boric acid is reacted with KHF2 (KF,HF), KBF4 salt is produced.
2KHF2 + H3BO3 → KBF4 + KOH + 2H2O
Potassium
Fluoroborate
(vii) When H3BO3 is heated with CaF2 and conc. H2SO4, boron trifluoride is formed
3CaF2 + 3H2SO4 + 2H3BO3 → 3CaSO4 → 3CaSO4 + 2BF3 +6H2O
For more help in Boric Acid click the button below to submit your homework assignment