Calculation Of Dissociation Energy Of Molecules
Calculation Of Dissociation Energy Of Molecules Assignment Help | Calculation Of Dissociation Energy Of Molecules Homework Help
Calculation of Dissociation Energy of Molecules
A study of the electronic band spectrum reveals that at end of the spectrum the distance of separation between the lines becomes smaller and smaller till ultimately the line spectrum vanishes and space of continuous emission starts. The frequency corresponding to the point where the line spectrum vanishes is the frequency necessary for dissociation of the molecule into atoms. Thus given by the vibrational-rotaional spectra but it can be used in evaluating the dissociation energies of the molecules.
Explanation of electronic band spectra and dissociation energies in terms of potential energy curves-Franck Condon principle.
The potential energy curve of a diatomic molecule is obtained by plotting the potential energy of the system virus antinuclear distance between the two approaching atoms. The curve obtained is of the shape shown in. However even at the minimum energy position (equilibrium position), represented by point X , the atoms are vibrating (so that the intern clear distance keeps on changing), therefore the potential energy keeps on oscillating about the minimum position . The vibration levels thus occupied by the system may be represented by horizontal lines and indicated by appropriate vibration quantum numbers. If sufficient energy is supplied so that the system occupies the vibration level represented by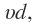 the corresponding intern clear dis- trance should become infinity i.e., the molecule should dissociate into atoms. However this usually does not happen because before this stage is reached, the electron jump s to the higher energy level i.e. , electronic transition takes place and the dissociation will take place only of the molecule in the higher energy level.
the corresponding intern clear dis- trance should become infinity i.e., the molecule should dissociate into atoms. However this usually does not happen because before this stage is reached, the electron jump s to the higher energy level i.e. , electronic transition takes place and the dissociation will take place only of the molecule in the higher energy level.
For more help in Calculation of Dissociation Energy of Molecules click the button below to submit your homework assignment
Explanation of electronic band spectra and dissociation energies in terms of potential energy curves-Franck Condon principle.
The potential energy curve of a diatomic molecule is obtained by plotting the potential energy of the system virus antinuclear distance between the two approaching atoms. The curve obtained is of the shape shown in. However even at the minimum energy position (equilibrium position), represented by point X , the atoms are vibrating (so that the intern clear distance keeps on changing), therefore the potential energy keeps on oscillating about the minimum position . The vibration levels thus occupied by the system may be represented by horizontal lines and indicated by appropriate vibration quantum numbers. If sufficient energy is supplied so that the system occupies the vibration level represented by
For more help in Calculation of Dissociation Energy of Molecules click the button below to submit your homework assignment