Chelates
Chelates Assignment Help | Chelates Homework Help
Chelates
Chelating ligand is a multidentate ligand which on coordination with the central metal atom results in ring formation. The complex thus produced is called a chelate and the process is called chelation. A chelate is formed if two or more donor atoms coordinate by the simultaneous use of two or more electron pairs to the same metal atom. An illustrative example may be,
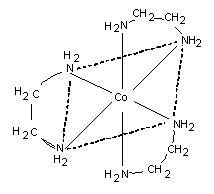
[Co(en)3]3+ or [Co(H2NCH2CH2NH2)3]3+.
All types of bidentate tridentate, tetradentate, pentadentate, and hexadentate ligands can act as chelating ligands and their complexes with metals are known as chelates. Normally cheated complexes are more stable than similar non-cheated complexes. In this example, ethylendiamine (en) occupies two coordination positions and thus behaves somewhat as if two molecules of ammonia are tied together.
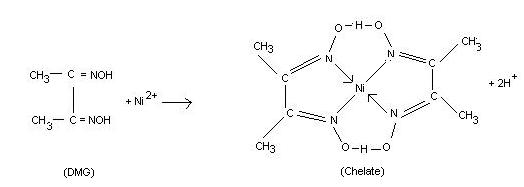
For example, EDTA (hexadentate ligand) is widely used for chelating metal ions. Deimethylglyoxime (DMG) and 8-hydroxyquinoline (oxine) are commonly used for the estimation of nickel and magnesium (or aluminum) ions in gravimetric analysis. These ions are chelated and form insoluble (in water) complexes which can be filtered easily.

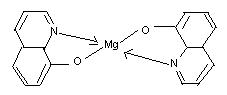
8-hydroxyquinoline (Chelate)
(oxine)
Some Special Features of Chelates :
(i) Chelating ligands form far more stable complexes than their condensate analogs . This is commonly known as Chelate effect.
As an example, we may consider the equilibrium constants of the following reactions:
Ni2+(aq) + 6NH3(aq) → [Ni(NH3)6]2+(aq)
log ß = 8.61
Ni2+(aq) + 3 en(aq) → [Ni(en)2+(aq)
log ß = 18.28
The complex (Ni(en)3]2+ in which three chelate rings re formed is about 10 times more stable than the other complex in which no ring is formed.
(ii) The stability of chelates depends on the number of atoms in the ring. Generally, chelates (which do not contain double bonds such as (‘en’) which form five-membered rings re very stable compounds. Ligands which contain double and (acac) form stable complexes containing is-membered rings.
(iii) Because of steric rasons, ligands with large groups form unstable compounds than the analogous ligands with smaller groups
For more help in Chelates click the button below to submit your homework assignment
[Co(en)3]3+ or [Co(H2NCH2CH2NH2)3]3+.
All types of bidentate tridentate, tetradentate, pentadentate, and hexadentate ligands can act as chelating ligands and their complexes with metals are known as chelates. Normally cheated complexes are more stable than similar non-cheated complexes. In this example, ethylendiamine (en) occupies two coordination positions and thus behaves somewhat as if two molecules of ammonia are tied together.

8-hydroxyquinoline (Chelate)
(oxine)
Some Special Features of Chelates :
(i) Chelating ligands form far more stable complexes than their condensate analogs . This is commonly known as Chelate effect.
As an example, we may consider the equilibrium constants of the following reactions:
Ni2+(aq) + 6NH3(aq) → [Ni(NH3)6]2+(aq)
log ß = 8.61
Ni2+(aq) + 3 en(aq) → [Ni(en)2+(aq)
log ß = 18.28
The complex (Ni(en)3]2+ in which three chelate rings re formed is about 10 times more stable than the other complex in which no ring is formed.
(ii) The stability of chelates depends on the number of atoms in the ring. Generally, chelates (which do not contain double bonds such as (‘en’) which form five-membered rings re very stable compounds. Ligands which contain double and (acac) form stable complexes containing is-membered rings.
(iii) Because of steric rasons, ligands with large groups form unstable compounds than the analogous ligands with smaller groups
For more help in Chelates click the button below to submit your homework assignment