Liquid Crystals
Liquid Crystals Assignment Help | Liquid Crystals Homework Help
Liquid Crystals
A large number of organic compounds with long chain molecules e.g.
Cholesterol acetate ( CH3COOC27H45 )
Cholestery benzoate (C6H5COOC27H45 ),
P-azoxyanisole
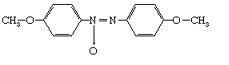
P-azoxy phentole
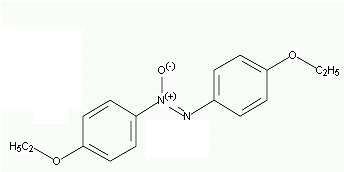
ammonium orate (C17H33COONH4) etc. are known which on heating melt sharply to give a milky (turbid) liquid which on further heating becomes clear again at a definite temperature.
The former temperature is called the transition point whereas the later is called the melting point (For example, for paxoxnisole transition point is 389 K and melting point is 408 K).
On cooling the clear liquid (melt), the reverse changes take place exactly at the same temperatures. The turbid liquids thus obtained are found to be anisotropic, a property possessed by crystalline substances (according to which the properties like refractive index, electrical conductivity are found to be different in different directions). Hence these turbid liquids are called liquid crystals. However, it is found that the molecules of a liquid crystal do not have the arrangement like that of a crystal lattice. Moreover except for a few properties (like refraction etc), they behave like true liquids e.g. they possess surface tension and have the property to flow. Hence in place of ‘liquid crystals’ certain other names have been proposed such as anisotropic liquids or Para crystals. The best name for the substances into his state is geomorphic state (which in Grid means intermediate state). However, in spite of all this the original name ‘liquid crystals’ is still frequently used.
For more help in Liquid Crystals click the button below to submit your homework assignment
Cholesterol acetate ( CH3COOC27H45 )
Cholestery benzoate (C6H5COOC27H45 ),
P-azoxyanisole

P-azoxy phentole

ammonium orate (C17H33COONH4) etc. are known which on heating melt sharply to give a milky (turbid) liquid which on further heating becomes clear again at a definite temperature.
The former temperature is called the transition point whereas the later is called the melting point (For example, for paxoxnisole transition point is 389 K and melting point is 408 K).
On cooling the clear liquid (melt), the reverse changes take place exactly at the same temperatures. The turbid liquids thus obtained are found to be anisotropic, a property possessed by crystalline substances (according to which the properties like refractive index, electrical conductivity are found to be different in different directions). Hence these turbid liquids are called liquid crystals. However, it is found that the molecules of a liquid crystal do not have the arrangement like that of a crystal lattice. Moreover except for a few properties (like refraction etc), they behave like true liquids e.g. they possess surface tension and have the property to flow. Hence in place of ‘liquid crystals’ certain other names have been proposed such as anisotropic liquids or Para crystals. The best name for the substances into his state is geomorphic state (which in Grid means intermediate state). However, in spite of all this the original name ‘liquid crystals’ is still frequently used.
For more help in Liquid Crystals click the button below to submit your homework assignment