Pauli Exclusion Principle
Pauli Exclusion Principle Assignment Help | Pauli Exclusion Principle Homework Help
Pauli Exclusion Principle
An electron in and atom is completely described by the four quantum number, n, 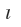 m and ms. The single electronic hydrogen atom occupies the west energy orbital with configuration 1si indicating n = 1,
m and ms. The single electronic hydrogen atom occupies the west energy orbital with configuration 1si indicating n = 1, 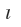 = 0, m = 0 and ms = +1/2. The next electronic helium atom enters the 1a orbital with opposite spin is supported by the atomic spectrum of helium. If the two electrons in He and like spins, then but the leering would have had identical set of four quantum number and the atomic spectrum of He would have been different form that observed for He. It is not so because electrons with the same spin repel each other and occupy different orbital’s and if they have to occupy the same orbital, they must have opposite spins. This is the result of Pauli Exclusion* principle,put forward by Pauli in1925.states:
= 0, m = 0 and ms = +1/2. The next electronic helium atom enters the 1a orbital with opposite spin is supported by the atomic spectrum of helium. If the two electrons in He and like spins, then but the leering would have had identical set of four quantum number and the atomic spectrum of He would have been different form that observed for He. It is not so because electrons with the same spin repel each other and occupy different orbital’s and if they have to occupy the same orbital, they must have opposite spins. This is the result of Pauli Exclusion* principle,put forward by Pauli in1925.states:
In an atom, a maximum of two electrons can occupy the same orbital.
As discussed above, the two electrons in the same orbital will have identical n,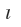 and m but must have different spins ( +1/2 and -1/2).
and m but must have different spins ( +1/2 and -1/2).
Pauli exclusion principle has no fundamental explanation but is an experimental fact. Although no proof of the exclusion principle exits yet nothing in nature could contradict it.
For more help in Pauli Exclusion Principle click the button below to submit your homework assignment
In an atom, a maximum of two electrons can occupy the same orbital.
As discussed above, the two electrons in the same orbital will have identical n,
Pauli exclusion principle has no fundamental explanation but is an experimental fact. Although no proof of the exclusion principle exits yet nothing in nature could contradict it.
For more help in Pauli Exclusion Principle click the button below to submit your homework assignment