Plancks Radiation Law
Plancks Radiation Law Assignment Help | Plancks Radiation Law Homework Help
Planck’s Radiation Law
To solve the above problem, Max Planck in 1990 put forward a bold assumption that the oscillator of black body cannot have nay amount of energy but can have only a discrete amount of energy depending upon the frequency of the oscillator. Planck thus summed up his result as under:
“Energy is emitted or absorbed not continuously but discontinuously in the form of packets of energy called gaunt. The energy of each quantum is given by the relation, E= hv, where v is the frequent of the radiation and h is called Planck’s constant. Thus the total energy emitted or absorbed is either unit quantum i.e. hv or a whole number multiple of hv i.e. equal to nhv.”
Based upon these concepts, Planck deduced expression the energy Eh radiated by a blackbody at wavelength h, which is as under:
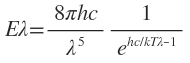
This expression called Planck’s radiation law. The significance of different symbols is given in Eqs. on the previous page.
It can be seen that both Wien’s law as well as Rayleigh’s law are simply particular cases of Planck’s radiation law.
(i) If T is small,
T is small, 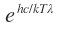 >>1 so that 1 in the denominator in above equation can be neglected. This gives
>>1 so that 1 in the denominator in above equation can be neglected. This gives
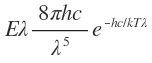
Which is Wien’s law.
(ii) If T is large,
T is large, 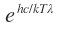 may be expanded by the exponential theorem,
may be expanded by the exponential theorem,
i.e.,
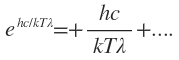
So that Planck’s radiation law equation reduces to the form
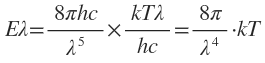
Which is Rayleigh-Jeans law.
For more help in Planck’s Radiation Law click the button below to submit your homework assignment
“Energy is emitted or absorbed not continuously but discontinuously in the form of packets of energy called gaunt. The energy of each quantum is given by the relation, E= hv, where v is the frequent of the radiation and h is called Planck’s constant. Thus the total energy emitted or absorbed is either unit quantum i.e. hv or a whole number multiple of hv i.e. equal to nhv.”
Based upon these concepts, Planck deduced expression the energy Eh radiated by a blackbody at wavelength h, which is as under:
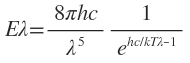
This expression called Planck’s radiation law. The significance of different symbols is given in Eqs. on the previous page.
It can be seen that both Wien’s law as well as Rayleigh’s law are simply particular cases of Planck’s radiation law.
(i) If
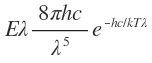
Which is Wien’s law.
(ii) If
i.e.,
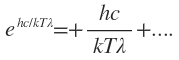
So that Planck’s radiation law equation reduces to the form
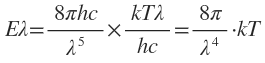
Which is Rayleigh-Jeans law.
For more help in Planck’s Radiation Law click the button below to submit your homework assignment