Principal Or Quantum Number
Principal Or Quantum Number Assignment Help | Principal Or Quantum Number Homework Help
Principal or Quantum Number
It raised form the solution of the radial part of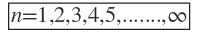
Theoretically there is no limit to the number of shells or levels but seventh shell (n=7) is the highest shell occupied by the know elements.
Principal quantum nuclear helps to determine the average distance of the electron form the uncles. A s n increases, the average distance of electron form nucleus increase.
The wave mechanical model recognizes that the energy values of the electron are quantized as postulated by Bohr. It further states that the three quantum number n alone does not give complete energy but designates the main energy level to which an electron belongs. The lowest energy state of an atom corresponds to n=1 and the energy increases with increasing value of n.
For more help in Principal or Quantum Number click the button below to submit your homework assignment