Schrodinger Wave Equation For Hydrogen Atom
Schrodinger Wave Equation For Hydrogen Atom Assignment Help | Schrodinger Wave Equation For Hydrogen Atom Homework Help
Schrodinger Wave Equation For Hydrogen Atom
For hydrogen atom, z = 1: therefore v =-e2and Schrödinger wave equation of H atom may be written as r
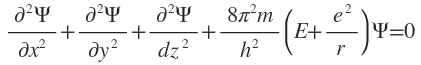
This famous time independent wave equation, describing the behavior of the electron in the hydro-gen atom, was arrived at by Schrodinger not by the above treatment but just by mathematical intuition. He wrote the equation in the above form on a piece of napkin while sitting in the Mensal (University mess). The a above treatment is in no way a proof of the Schrödinger wave equation-in fact Schrödinger wave equation is not derivable-but it merely shows that the equation results form the assumptions:
(i) that the behavior of an electron in an atom is analogous to a system of standing wave; and
(ii) de Broglie relationship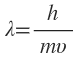 describes the wavelength of electron.
describes the wavelength of electron.
Justification for the above equation ofr hydrogen atom is found in the fact that the solution of equation gives values of energy which agree well with those obtained experimentally from atomic spectra or calculated form the equation given by Bohr for his model of the atom:
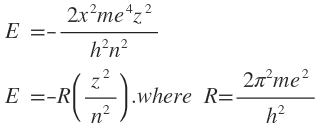
R is a constant known as Rhydberg constant.
For hydrogen atom (Z=1), the lowest energy state (n-1) equals to – R which has a value of 1312 KJ mol-1.
That is, Schrodinger wave approach leads to exactly the same allowed energies as those previously deduced form the Bohr model. Besides that the most probable radius for electron in hydrogen atom form wave mechanics is also the same as previously determined Bohre radius (0.529 A) from equation for Bohr model
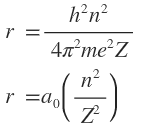
or
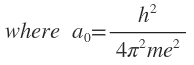
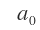 is a constant known as Bohr radius. For hydrogen (Z=1), allowed values of r are 1a0,4a0,9a0 for n=1, 2 or 3, respectively.
is a constant known as Bohr radius. For hydrogen (Z=1), allowed values of r are 1a0,4a0,9a0 for n=1, 2 or 3, respectively.
a that is. Bohr radius has a value of 0.529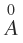 .
.
For more help in Schrodinger Wave Equation For Hydrogen Atom click the button below to submit your homework assignment
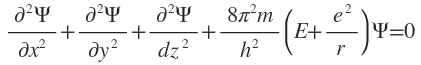
This famous time independent wave equation, describing the behavior of the electron in the hydro-gen atom, was arrived at by Schrodinger not by the above treatment but just by mathematical intuition. He wrote the equation in the above form on a piece of napkin while sitting in the Mensal (University mess). The a above treatment is in no way a proof of the Schrödinger wave equation-in fact Schrödinger wave equation is not derivable-but it merely shows that the equation results form the assumptions:
(i) that the behavior of an electron in an atom is analogous to a system of standing wave; and
(ii) de Broglie relationship
Justification for the above equation ofr hydrogen atom is found in the fact that the solution of equation gives values of energy which agree well with those obtained experimentally from atomic spectra or calculated form the equation given by Bohr for his model of the atom:
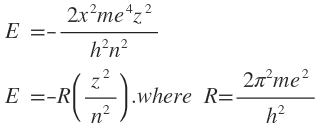
R is a constant known as Rhydberg constant.
For hydrogen atom (Z=1), the lowest energy state (n-1) equals to – R which has a value of 1312 KJ mol-1.
That is, Schrodinger wave approach leads to exactly the same allowed energies as those previously deduced form the Bohr model. Besides that the most probable radius for electron in hydrogen atom form wave mechanics is also the same as previously determined Bohre radius (0.529 A) from equation for Bohr model
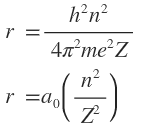
or
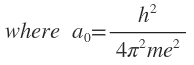
a that is. Bohr radius has a value of 0.529
For more help in Schrodinger Wave Equation For Hydrogen Atom click the button below to submit your homework assignment