Shape Of S Orbitals
Shape Of S Orbitals Assignment Help | Shape Of S Orbitals Homework Help
Shape of s-orbitals
As already stated the expression for angular wave function for 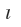 = 0 that is s-orbital, is not dependent on either of the angles
= 0 that is s-orbital, is not dependent on either of the angles 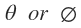 . In other words, the angular part of the wave function is spherically symmetrical. That is, the probability of finding the electron is equal in all directions. Remembering that atoms are three dimensional, the probability surface diagram of s-orbital spherical. The two dimensional method of illustrating spherical distribution of s-orbital spherical distribution of s-orbital’s is to draw a circle as shown in . It may be borne in mind that the radius of the circle is almost2-4 times the Bohr radius or “most probable” radius. For example, the 1s-orbital in hydrogen atom may be taken as a sphere with radius of the order of 1-2A.
. In other words, the angular part of the wave function is spherically symmetrical. That is, the probability of finding the electron is equal in all directions. Remembering that atoms are three dimensional, the probability surface diagram of s-orbital spherical. The two dimensional method of illustrating spherical distribution of s-orbital spherical distribution of s-orbital’s is to draw a circle as shown in . It may be borne in mind that the radius of the circle is almost2-4 times the Bohr radius or “most probable” radius. For example, the 1s-orbital in hydrogen atom may be taken as a sphere with radius of the order of 1-2A.

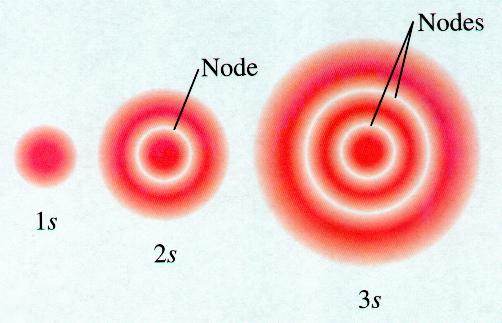
It we look at the radial distributing curve for 2s-orbital , we find two regions of high probability separated by a node at r = r. If we have to draw a dot density diagram for 2s on the lines similar to 1s then for 2s shows two regions of higher dot density separated by a spherical nodal surface (shown as dotted circle) at distance r1 form the nucleus. Such diagrams showing nodal surfaces will be avoided because by doing so we are extending the electron as a particle in one region and then to explain its motion to the other region through an pirate because such a wave can exist simultaneously on but sides of mode.
Dot representation electron density in 2s orbital
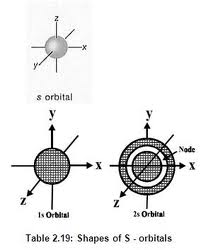
Even through the s-orbital’s are usually depicted in two dimensions in the form of a circle as shown above, spatial rep-recantation of 1a and 2s orbital’s is given in.
Spatial representation of 1s and 2s-orbitals
For more help in Shape of s-orbitals click the button below to submit your homework assignment

It we look at the radial distributing curve for 2s-orbital , we find two regions of high probability separated by a node at r = r. If we have to draw a dot density diagram for 2s on the lines similar to 1s then for 2s shows two regions of higher dot density separated by a spherical nodal surface (shown as dotted circle) at distance r1 form the nucleus. Such diagrams showing nodal surfaces will be avoided because by doing so we are extending the electron as a particle in one region and then to explain its motion to the other region through an pirate because such a wave can exist simultaneously on but sides of mode.

Even through the s-orbital’s are usually depicted in two dimensions in the form of a circle as shown above, spatial rep-recantation of 1a and 2s orbital’s is given in.

For more help in Shape of s-orbitals click the button below to submit your homework assignment