Significance Of Wave Function
Significance Of Wave Function Assignment Help | Significance Of Wave Function Homework Help
Significance of Wave Function
The symbol 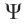 occurs in the wave equation as the amplitude function which needs explanation for better understanding of the electron behavior. Does the amplitude function
occurs in the wave equation as the amplitude function which needs explanation for better understanding of the electron behavior. Does the amplitude function 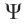 have any physical significance like the one we attach to other waves? The straight-forward answer to this equation is No. That is
have any physical significance like the one we attach to other waves? The straight-forward answer to this equation is No. That is 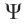 has only mathematical significance an do not attach any physical significance to
has only mathematical significance an do not attach any physical significance to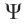 ,.
,.
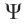 as a wave function must obey the following condition if it is to give meaningful results:
as a wave function must obey the following condition if it is to give meaningful results:
(i)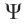 must be single valued, i.e. it must have only one definite value at a particular point in space.
must be single valued, i.e. it must have only one definite value at a particular point in space.
(ii)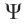 must be continuous at all points in space, i.e. there must not be sudden changes in the values of
must be continuous at all points in space, i.e. there must not be sudden changes in the values of 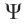 when its variables are changed.
when its variables are changed.
(iii)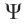 must have a finite value over the space that the electron can occupy.
must have a finite value over the space that the electron can occupy.
(iv)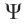 must be normalized, i.e., the probability of finding the electron in the space under consideration must be unity.
must be normalized, i.e., the probability of finding the electron in the space under consideration must be unity.
(v)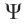 must become zero at infinity.
must become zero at infinity.
This means that of all the values of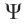 obtained by solving Schrödinger wave equation, only those values of
obtained by solving Schrödinger wave equation, only those values of 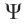 are accepted which obey the above condition. Thus only acceptable values of
are accepted which obey the above condition. Thus only acceptable values of 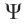 have significance.
have significance.
The significant values of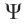 are called Eigen function or wave function. Eigen function give significant values of the total energy of Electron (E) called Eigen values. These Eigen values correspond to the discrete energy levels as postulated by Niels Bohr.
are called Eigen function or wave function. Eigen function give significant values of the total energy of Electron (E) called Eigen values. These Eigen values correspond to the discrete energy levels as postulated by Niels Bohr.
For more help in Significance of Wave Function click the button below to submit your homework assignment
(i)
(ii)
(iii)
(iv)
(v)
This means that of all the values of
The significant values of
For more help in Significance of Wave Function click the button below to submit your homework assignment