Space Lattice And Unit Cell
Space Lattice And Unit Cell Assignment Help | Space Lattice And Unit Cell Homework Help
Space Lattice and Unit Cell
So far we have been discussing only the external shape of the crystal. On studying the internal structure of the crystal, it is found the participles (i.e. ions, atoms or molecules) constituting the crystalline substance are arranged in a regular fashion within the crystal in the three dimensional space, For example, the arrangement of particles (represented by black circles) for a cubic crystal is shown in Fig.
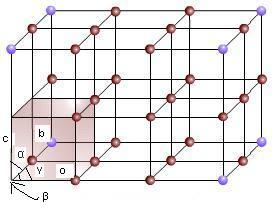
Space lattice and unit cell
The regular arrangement of points (i.e. ions, atoms, or molecules constituting the crystal) in the three dimensional space within the crystal is called the space lattice.
If a big-sized crystal is broken more and more, ultimately a stage is reached when we get the smallest possible crystal. if it is broken further, it will break to give the consistent particles. i.e., ions, atoms or molecules. For example, if a big cubic crystal is broken, the smallest crystal obtained will be a small cube, as represented by thick line in above. This small cubic crystal has all the elements of symmetry a possessed by the big cubic crystal. Moreover it is evident the complete lattice has been obtained by the repartition of this smallest unit in different directions.
The smallest portion of the complete space lattice which when repeated over and again in different directions produces the complete space lattice is called the unit cell.
For more help in Space Lattice and Unit Cell click the button below to submit your homework assignment
Space lattice and unit cell
The regular arrangement of points (i.e. ions, atoms, or molecules constituting the crystal) in the three dimensional space within the crystal is called the space lattice.
If a big-sized crystal is broken more and more, ultimately a stage is reached when we get the smallest possible crystal. if it is broken further, it will break to give the consistent particles. i.e., ions, atoms or molecules. For example, if a big cubic crystal is broken, the smallest crystal obtained will be a small cube, as represented by thick line in above. This small cubic crystal has all the elements of symmetry a possessed by the big cubic crystal. Moreover it is evident the complete lattice has been obtained by the repartition of this smallest unit in different directions.
The smallest portion of the complete space lattice which when repeated over and again in different directions produces the complete space lattice is called the unit cell.
For more help in Space Lattice and Unit Cell click the button below to submit your homework assignment