Spin Quantum Number
Spin Quantum Number Assignment Help | Spin Quantum Number Homework Help
Spin Quantum Number
In 1896, Zeeman observed that spectral lines in the atomic spectrum get split under the influence of a strong magnetic field. This phenomenon, called the Zeeman effect, is attributed to the orientation of the components of the angular momentum with respect to the external magnetic field and is given by 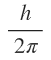 . Where m = magnetic quantum number.
. Where m = magnetic quantum number.
Later on , it was observed in the atomic spectra of alkali metals that the spectra lines which were earlier considered to be single lines have actually come out to be narrow doublets (two lines quite close together).An explanation to these doublet lines was offered by Uhlenbeck and Goudmit in 1925. They proposed that the electron (besides its orbital motion)is also associated with rotation about its won axis (spinning). Axial spin of the electron is associated with spin angular momentum whose magnitude is given by m,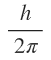 where ms is called the spin quantum number. The spin quantum number can have values
where ms is called the spin quantum number. The spin quantum number can have values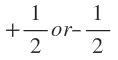 which are thought to arise from the direction of spin (clockwise or anticlockwise). It is also designated as
which are thought to arise from the direction of spin (clockwise or anticlockwise). It is also designated as 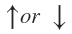 Thus, the description of an electron is an atom in term of three quantum number (described earlier) is not complete and , in fact, quantum number n.
Thus, the description of an electron is an atom in term of three quantum number (described earlier) is not complete and , in fact, quantum number n.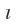 , m and ms :are required to describe the electron in an atom completely.
, m and ms :are required to describe the electron in an atom completely.
For more help in Spain Quantum Number click the button below to submit your homework assignment
Later on , it was observed in the atomic spectra of alkali metals that the spectra lines which were earlier considered to be single lines have actually come out to be narrow doublets (two lines quite close together).An explanation to these doublet lines was offered by Uhlenbeck and Goudmit in 1925. They proposed that the electron (besides its orbital motion)is also associated with rotation about its won axis (spinning). Axial spin of the electron is associated with spin angular momentum whose magnitude is given by m,
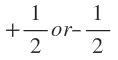 which are thought to arise from the direction of spin (clockwise or anticlockwise). It is also designated as
which are thought to arise from the direction of spin (clockwise or anticlockwise). It is also designated as 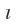 , m and ms :are required to describe the electron in an atom completely.
, m and ms :are required to describe the electron in an atom completely.For more help in Spain Quantum Number click the button below to submit your homework assignment