Structures Of Borates
Structures Of Borates Assignment Help | Structures Of Borates Homework Help
Structures Of Borates
It is very difficult to predict the structures of the borate anions from the stoichiometry of the complex borates. Anhydrous borates are prepared by fusing boric acid and metal oxides. The hydrated borates may be cryallized form aqueous solutions. The general composition of borates : KB5O8.4H2O. CaB2O4, Na,B4O7.10H2O gives very little information about the strucutes of the borate anions. They are generally cyclic or linear polymers formed by linking together of BO3 and/or BO4 units through shard oxygen atoms. Bortes containing one or more than one boron atom (upto 5B atoms) or polydimentsional networks (glasses) are known to exist. They mainly consist of :
(i) Boron atom linked either to three oxygen atoms (BO3 unit) or four oxygen atoms (BO4 tetrahedron).
(ii) Polynuclear anions are formed by corner sharing of boron-oxygen triangles or tetrahedrons.
(iii) These units can be prorogated to various extents.
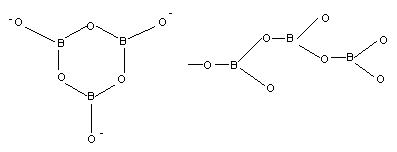
Ring (K3B2O4) Chain (CaB3O6)
It may be noted that contrast to borates, the carbonate ion does not form polymeric strucutes. This may probably be attributed to the formation of strong C-O π bonds.
Borax, which is normally written as Na2B4O7.10H2O contains tetranuclear units [B4O5(OH)4]2- comprising of BO4 and BO3 units. Borax should therefore be formulated as Na2[B4O5(OH)4]4H2O.
For more help in Structures Of Borates click the button below to submit your homework assignment
(i) Boron atom linked either to three oxygen atoms (BO3 unit) or four oxygen atoms (BO4 tetrahedron).
(ii) Polynuclear anions are formed by corner sharing of boron-oxygen triangles or tetrahedrons.
(iii) These units can be prorogated to various extents.
It may be noted that contrast to borates, the carbonate ion does not form polymeric strucutes. This may probably be attributed to the formation of strong C-O π bonds.
Borax, which is normally written as Na2B4O7.10H2O contains tetranuclear units [B4O5(OH)4]2- comprising of BO4 and BO3 units. Borax should therefore be formulated as Na2[B4O5(OH)4]4H2O.
For more help in Structures Of Borates click the button below to submit your homework assignment