Transition Metal Hydrides
Transition Metal Hydrides Assignment Help | Transition Metal Hydrides Homework Help
Transition Metal Hydrides
Transition metal is covalently bonded to hydrogen in transition metal hydrides which generally have a formulation of the type MHxLy where M = transition metal and L = carbon monoxide CO, organophosphine R3 P, cyclopentadieno C5H5-. etc., type of ligands which can act both as o-donor as well as π- acceptor ligands* M 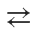 : L Besides being covalent , the transition metal-hydrogen bond is longer than normal. Some examples of these hydrides are :
: L Besides being covalent , the transition metal-hydrogen bond is longer than normal. Some examples of these hydrides are :
K3 [ H-Co(CN)5 ], (C5H5)2MoH2, (C5H5)2Re-H, (Et3 P)2(CI)Pt-H, HMn(CO)5
Preparation of Transition metal hydrides
Transition metal hydrides are not many in number. Some of the important ones are prepared as follows:
(i) MOCI5 + 3NaC5H5** + 2NaBH4 → (C5H5)2MoH2 + 5NaCI + B2H6 + 1 C10H10
2
(ii) (C5H5)Fe(CO)2CI + NaBH4 → (C5H5)Fe(CO2)H + NaCI + BH3
(iii) Pt(PR3)2 CI2 + LiAIH4 → (PR3)2Pt(CI)H + LiCI + AIH3
Prosperities of Transition metal hydrides
Most of these compounds are very reactive solids. For example, (C5H5)2 MoH2 reacts with chloroform t form dichloro derivative.
CHCI3
(C5H5)2 MoH2 → (C5H5)2MoCI2 .
(R3P)2Pt(CI)H reacts with ethylene to form ethyl derivative.
(R3P)2(CI)Pt-H + C2H4 → (R3P)2 (CI)Pt-C2H5
For more help in Transition Metal Hydrides click the button below to submit your homework assignment
K3 [ H-Co(CN)5 ], (C5H5)2MoH2, (C5H5)2Re-H, (Et3 P)2(CI)Pt-H, HMn(CO)5
Preparation of Transition metal hydrides
Transition metal hydrides are not many in number. Some of the important ones are prepared as follows:
(i) MOCI5 + 3NaC5H5** + 2NaBH4 → (C5H5)2MoH2 + 5NaCI + B2H6 + 1 C10H10
2
(ii) (C5H5)Fe(CO)2CI + NaBH4 → (C5H5)Fe(CO2)H + NaCI + BH3
(iii) Pt(PR3)2 CI2 + LiAIH4 → (PR3)2Pt(CI)H + LiCI + AIH3
Prosperities of Transition metal hydrides
Most of these compounds are very reactive solids. For example, (C5H5)2 MoH2 reacts with chloroform t form dichloro derivative.
CHCI3
(C5H5)2 MoH2 → (C5H5)2MoCI2 .
(R3P)2Pt(CI)H reacts with ethylene to form ethyl derivative.
(R3P)2(CI)Pt-H + C2H4 → (R3P)2 (CI)Pt-C2H5
For more help in Transition Metal Hydrides click the button below to submit your homework assignment