Determination Of Transport Number
Determination Of Transport Number Assignment Help | Determination Of Transport Number Homework Help
Determination of Transport Number
(i) Electrolytes in which no chemical action takes place at the electrodes
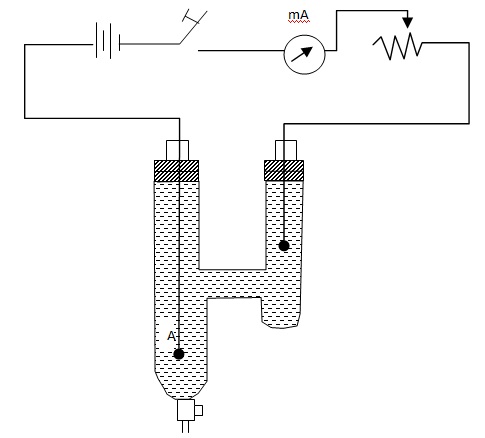
A and C are plantinum electrodes arranged at the bottom of a H-shaped glass vessel. The apparatus is filled with silver nitrate solution of known concentration. The electrolyte can be drawn out of each tube through a stopcock at its bottom. The platinum electrodes are connected to a 110 V D.C. supply through a milliammeter and rheostat.A suitable current is passed through the electrolyte for a known time. Then, small quantities of electrolyte are withdrawn from the anode and cathode limbs into separate breakers. Their concentrations are estimated by analysis. Since the initial concentration of the electrolyte
(ii) Electrolytes in which chemical action takes place at the electrodes
The apparatus is a H-shaped glass vessel. A and C are anode and cathode of silver. The electrolyte is silver nitrate in water. The shorter limb contains the cathode and its lower end is closed. The longer limb contains the anode and has a stop cock at its bottom. The anode is arranged close to the bottom of the tube.The cathode is weighted accurately (w1). The apparatus is filled with silver nitrate solution of known concentration C1. Then current is passed for a known interval of time. The cathode is weighed again accurately (w2). A small quantity of electrolyte is withdrawn from the anode region and its concentration is determined by analysis. There will be an increase of concentration. Let C2 be the present concentration.
Increase in concentration = C = C2 – C1.
Increase in mass of cathode = m = (w2 – w1)
Let u and v be the velocities of the sliver ion (cation) and nitrate ion (anion) respectively during electrolysis. Nitrate ions from the cathode side migrate to the anode side at the rate of v ms-1 leaving v silver ions near cathode. Similarly, silver ions from the anode side migrate to the cathode side at the rate of u ms-1. Hence at the cathode side, the rate of increase of silver ions is (u + v). These silver ions on delivering the charges to the cathode are deposited on the cathode as silver atoms. Thus the increase in the mass of the cathode is proportional to (u + v).
m ∞ (u + v) … (1)
Similarly, the rate of increase of nitrate ions in the anode side is proportional to (u + v). They combine with (u + v) silver atoms of the anode, forming (u + v) silver nitrate molecules in the anode space. Thus in the anode side, the net increase of silver nitrate molecules is v. Hence the increase in concentration of silver nitrate solution in the anode side is proportional to v.
C ∞ v … (2)
The transport number of the nitrate ion is
na = v/u + v = C/ m … (3)
The transport number of the silver ion = nc = 1 – na … (4)
Thus the transport numbers of the silver and nitrate ions under on applied field can be calculated.
For more help in Determination of Transport Number click the button below to submit your homework assignment