Equivalent Conductivity
Equivalent Conductivity Assignment Help | Equivalent Conductivity Homework Help
Equivalent Conductivity
Equivalent conductivity of an electrolyte is defined as the ratio of the specific conductivity to the concentration of the electrolytic solution in gram-equivalent per c.c.If λ is the equivalent conductivity, then λ = σ / C where σ is the specific conductivity and C is the concentration. If one gram equivalent of the solute is dissolved in v.c.c of the solution, then v = 1/C. v is called the dilution.
Equivalent conductivity = λ = σ/C = σv
Molecular conductivity (μ) is defined as the product of the specific conductivity and the volume v1 c.c containing a gram molecule (Mole) of the solute.
μ = σ v1.
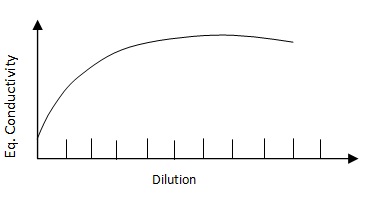
The equivalent and molecular conductivities increase as the dilution increases. For very dilute solution (infinite dilution), the equivalent conductivity becomes constant (λ∞).
The value of equivalent conductivity is determined at different dilutions. A graph is plotted representing λ (equivalent conductivity) on the Y axis and the corresponding value of dilution which is 1/C on the axis. A curve is obtained. The value of λ∞ (equivalent conductivity at infinite dilution) is thus terminated.
For more help in Equivalent Conductivity click the button below to submit your homework assignment