Applications Of Electrolysis
Applications Of Electrolysis Assignment Help | Applications Of Electrolysis Homework Help
Applications of Electrolysis
1. Extraction and Refining of Metals:
In the process of refining of metals the impure metal extracted from its ore is made anode, the salt of the metal is made electrolyte. Pure metal deposits on the cathode.
At regular intervals, the deposit is stripped from the cathode and the anode is replaced. For example copper extracted from its ore, known s blister copper is 98% to 99% pure and has high resistively but the copper required for electrical applications must have a minimum purity of 99.92%. Copper of purity as high as 99.95% is obtained from blister copper by electrolytic process using copper sulphate solution as electrolyte. Zinc and other metals are also purified in similar fashion.
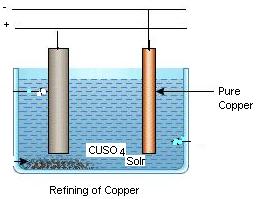
At regular intervals, the deposit is stripped from the cathode and the anode is replaced. For example copper extracted from its ore, known s blister copper is 98% to 99% pure and has high resistively but the copper required for electrical applications must have a minimum purity of 99.92%. Copper of purity as high as 99.95% is obtained from blister copper by electrolytic process using copper sulphate solution as electrolyte. Zinc and other metals are also purified in similar fashion.
2. Production of chemicals:
Many chemicals, such as caustic soda, chlorine, potassium permanganate, ammonium per-sulphate, hydrogen and oxygen etc. are produced by electrolysis on a large scale. The most important is production of caustic sods by electrolysis of brine. During the process of electrolysis of brine, chlorine is given off. at one pole (at anode) and hydrogen at the other (at cathode) leaving caustic soda n the cell. The brine solution becomes more and more rich caustic sods. The by-products of hydrogen and chlorine in no way are less important. These by-products are further used to produce hydrochlorn-acid (HCI). If chlorine and hydrogen re not separated, hydrochloride, chlorate or peri chlorates are produced.
3. Electrolytic Reduction of Metals from their Compounds:
It is employed for obtaining several metals for example aluminum is obtained from bauxite by this method. High grade bauxite contains up to 70% aluminum oxide chemically combined with water forming a hydrated oxide. Besides it the ore also contains silica and iron oxide. First pare aluminum oxide is obtained by suitable treatment and then pure aluminum is obtained by reducing aluminum oxide. The aluminum obtained by this process is pure up tot 99.5 percent.
4. Electroplating:
This is a process of cover articles made of cheap metals by a thin covering of precious such as iron with nickel or chromium of silver or gold. The article made of one metal may be electroplated to cover it by another metal with one or more of the following aims :

(i) decoration (ii) protection against corrosion (iii) for reconstruction or repair (iv) as an intermediate manufacturing process.
The object to be electroplated is thoroughly cleaned, polished, degreased and arranged as a cathode in a voltammeter containing anode and electrolyte of the metal to be deposited. To give even deposits all over the object either the cathode must be surrounded by set of anodes or cathode must be rotated at uniform speed. The chemical nature of the electrolyte used depends upon the type of plating. For gold or silver plating, the electrolyte is always alkaline, for nickel or copper plating. It is usually acid.
(i) decoration (ii) protection against corrosion (iii) for reconstruction or repair (iv) as an intermediate manufacturing process.
The object to be electroplated is thoroughly cleaned, polished, degreased and arranged as a cathode in a voltammeter containing anode and electrolyte of the metal to be deposited. To give even deposits all over the object either the cathode must be surrounded by set of anodes or cathode must be rotated at uniform speed. The chemical nature of the electrolyte used depends upon the type of plating. For gold or silver plating, the electrolyte is always alkaline, for nickel or copper plating. It is usually acid.
5. Electro-forming:
Reproduction of objects by electro-deposition on some sort of a mould or form is known as electro-forming. This is another application of electro-deposition.
In the reproduction of coins, medals, engravings and the like, a mould is first made by impressing the object, say, in wax. The surface of the wax, which bears exact impressions of the object, is coated by powdered graphite in order to make conducting. The mould is then dipped in an electro-forming cell as a cathode. After obtaining coating of desired thickness, the article is removed and the wax core is melted out of the metal shell.
The process is employed in the manufacture of gramophone records, in which the original recording is done on a record of wax composition. This mould is then coated with powdered gold to make it conducting and dipped into a blue vitriol electrolyte as cathode. The solutions kept saturated by using a copper anode. The plating of copper on to the wax record produces a negative master plate which is used to stamp a large number of shellac discs.
For more help in Applications of Electrolysis click the button below to submit your homework assignment
In the reproduction of coins, medals, engravings and the like, a mould is first made by impressing the object, say, in wax. The surface of the wax, which bears exact impressions of the object, is coated by powdered graphite in order to make conducting. The mould is then dipped in an electro-forming cell as a cathode. After obtaining coating of desired thickness, the article is removed and the wax core is melted out of the metal shell.
The process is employed in the manufacture of gramophone records, in which the original recording is done on a record of wax composition. This mould is then coated with powdered gold to make it conducting and dipped into a blue vitriol electrolyte as cathode. The solutions kept saturated by using a copper anode. The plating of copper on to the wax record produces a negative master plate which is used to stamp a large number of shellac discs.
For more help in Applications of Electrolysis click the button below to submit your homework assignment